The valence shell of an atom represents a band of energy levels and that the valence electrons are confined to that band. When an electron acquires enough additional energy, it can leave the valence shell, become a free electron, and exist in what is known as the conduction band.
The difference in energy between the valence band and the conduction band is called an energy gap or band gap. This is the amount of energy that a valence electron must have in order to jump from the valence band to the conduction band. Once in the conduction band, the electron is free to move throughout the material and is not tied to any given atom.
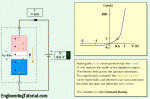
The below figure shows energy diagrams for insulators, semiconductors, and conductors. The energy gap or band gap is the difference between two energy levels and is “not allowed” in quantum theory. It is a region in insulators and semiconductors where no electron states exist. Although an electron may not exist in this region, it can “jump” across it under certain conditions. For insulators, the gap can be crossed only when breakdown conditions occur—as when a very high voltage is applied across the material.
The band gap is illustrated in Figure 1–(a) for insulators. In semiconductors the band gap is smaller, allowing an electron in the valence band to jump into the conduction band if it absorbs a photon. The band gap depends on the semiconductor material. This is illustrated in Figure 1–(b). In conductors, the conduction band and valence band overlap, so there is no gap, as shown in Figure 1–(c). This means that electrons in the valence band move freely into the conduction band, so there are always electrons available as free electrons.
![]()