Sulfur Hexafluoride Gas (SF6) Properties
- Heavy, chemically inert, non toxic
- No poisonous effect on the human body but decomposition products are poisonous
- Color less and odor less
- It is gaseous at normal room temperature and pressure
- Density is about 6.6g/l at 20oC (5 times denser than air)
- Critical temperature is at 45.6oC and can be liquefied by compression
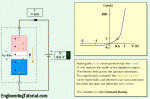
- Very good insulant with high dielectric strength
- SF6 gas is electo-negitive ( tends to attract the free electrons and has the arc quenching property). Because of this main reason SF6 gas is used for arc quenching and insulation medium in circuit breakers.
- The gas is highly stable
- Unlike solid insulation materials, electrical breakdown of sf6 gas does not result in permanent deterioration of its properties.
- Decomposition occurs on the exposure to the electric arc. (Disassociation products will be SF2 and SF4 lower order fluorides)